Heterogeneous fluid inclusion trapping:
Explaining the formation of CO2-only fluid
inclusions
K. Burlinson April 2018, November 2019
Introduction
There has been much confusion in the literature
over the observation of completely CO2 filled fluid
inclusions within quartz, lacking any visible water phase. In
many cases the authors have concluded that the inclusions must
have formed from a non-aqueous CO2 fluid. But
this is nonsense because everyone has forgotten that you must
first have deposition of silica in order
to trap a fluid inclusion and silica is not transported in or
deposited from pure CO2 fluids. These CO2
filled inclusions can be formed by heterogeneous trapping from
aqueous fluids; there is no need to propose unsubstantiated
silica transport and deposition from non-aqueous CO2
fluids!
Because of the extensive immiscibility of CO2
and H2O fluids, low temperature trapping below 300 °C
and disproportional trapping, the fluid
inclusion assemblages
(FIAs) may leave no trace of either of the 2 fluid
phases, which many authors fail to understand. An aqueous CO2
bearing hydrothermal fluid which is homogeneous at high
temperatures will separate into a mixture of 2 immiscible fluids
as the temperature reduces. At the trapping temperatures
typically observed in hydrothermal gold deposits, even parent
fluids with only a small content of CO2 will separate
into a heterogeneous mixture of these immiscible fluids, one
aqueous and the other carbonic. But depending on the rate of
silica deposition and conditions within the fluid only one of
the heterogeneous fluids may be trapped, leading to the false
assumption of a homogeneous single phase fluid system. It is
well known that inclusions
trapped from a heterogeneous fluid do not trap the 2 (or more)
(possibly immiscible) phases in proportion to their abundance.
By understanding the immiscibility of CO2-H2O
fluids it is simple to explain the trapping of CO2-only
fluid inclusions from a heterogeneous fluid system in which the
aqueous phase transported the essential silica, and probably the
elements of economic interest such as gold. The claims that gold
and silica are transported in pure CO2 fluids are
unsubstantiated and false.
Definitions
This discussion uses the term "heterogeneous
trapping" to refer to inclusion trapping from an aqueous CO2
bearing fluid which has become immiscible at the solvus and has
separated into two fluids, a conjugate-pair of fluids with
compositions determined by the solvus curve. This results in two
fluids, from which the individual fluid inclusions trapped would
be comprised, in theory, of either or both of the aqueous
or the carbonic conjugate fluid. I do not discuss mere
mechanical mixtures of fluids of random composition as it is
improbable that mechanical mixtures of immiscible fluids can be
trapped within individual fluid inclusions as they would not
remain stable enough for the long time period required to be
encased in the slowly depositing silica.
Summary
- CO2 rich aqueous hydrothermal fluids
frequently ex-solve into 2 conjugate fluids, one aqueous
and one almost entirely CO2 because of
immiscibility as the pressure, temperature and salinity
varies.
- The CO2-rich fluid from which inclusions are
trapped is frequently heterogeneous and should not be
assumed to be homogeneous despite appearances in thin
sections.
- The 2 conjugate fluids may not both be trapped because
the gaseous and aqueous fluids are trapped as inclusions
by completely different, mutually opposing mechanisms.
- This selective trapping is often so extreme that only
one of the 2 fluids occurs in the observed fluid inclusion
assemblages, leading to false assumptions of a homogeneous
fluid.
- The criteria proposed by Ramboz et al. (1982) to
identify heterogeneous trapping in observed assemblages
can prove heterogeneous trapping but are too restrictive
and do not disprove it.
- As CO2 ex-solves from the fluid, quartz
solubility increases and fluid inclusion trapping ceases
until quartz saturation is re-established at much lower
temperatures.
- These complications cause serious misinterpretation of
the original fluid composition when the interpretation
fails to allow for selective and dis-proportional trapping
of inclusions from heterogeneous fluids and assumes that
heterogeneous fluids can be identified by thin section
observations.
Explanation
The diagram below shows the phase relations of a
CO2 - H2O system with Temperature and
Pressure. (XCO2 is the mole fraction of CO2)
The solvus curves of the miscibility boundary are shown at 4
different pressures. The data is from Tödheide and Franck
(1963). (Yes, the isobaric solvus curves do intersect and cross.
The CO2-H2O
system is complex and non-linear with pressure!) If there is any NaCl present, the immiscibility
is greatly increased. Clearly immiscibility is a major
issue in such fluids and will affect many gold bearing
hydrothermal fluid systems, as they frequently contain CO2.
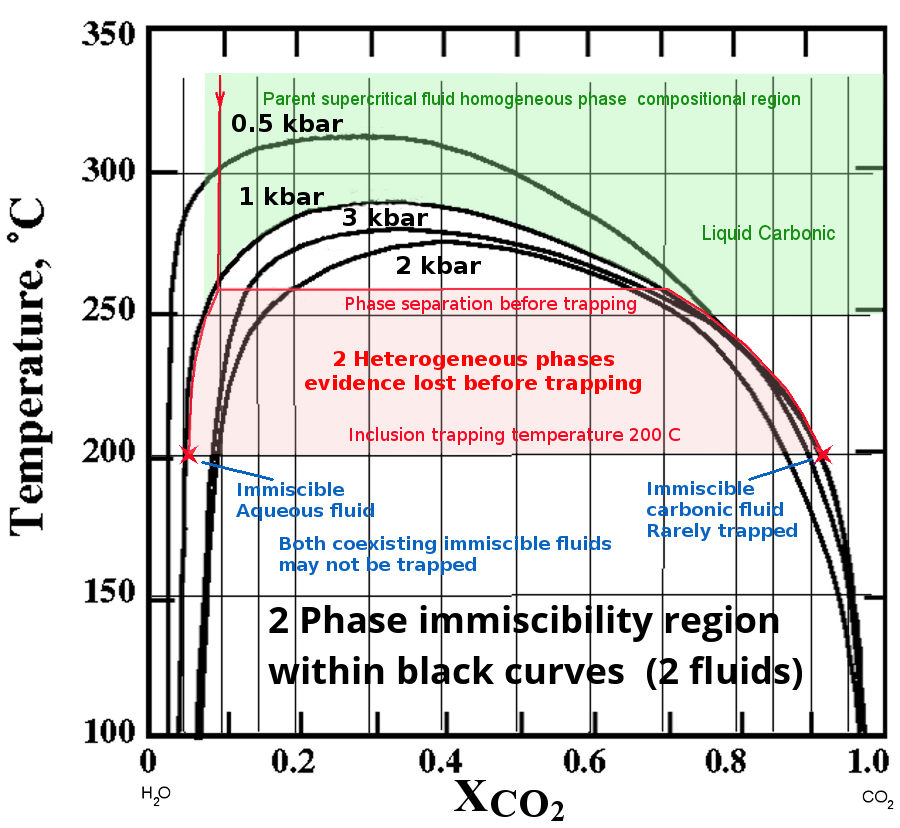
At a pressure of 1 Kbar, the fluid in the region
shown in green is a homogeneous single phase. If we consider an
initial fluid with just 0.1 mole fraction of CO2 at
350°C, as it cools it will follow the path of the red line down
until it intersects the solvus at about 260°C. Here it will
separate into two immiscible phases, one dominantly aqueous
(liquid, as this is below the critical point temperature of
water and at high pressure) and the other a water
saturated CO2 phase (a super-critical fluid because
it is above the critical point temperature of CO2 and
at high pressure). But inclusion trapping does not occur
until silica saturation is reached, assumed in this
example to be at 200°C and shown as 2 red crosses in this
diagram. Note that the solubility of silica increases as CO2
is ex-solved from the solution, which prevents the deposition of
silica and formation of fluid inclusions until the temperature
is considerably reduced (discussion below).
From 260°C down to 200°C, both fluid phases evolve and coexist
as a mixture in an open fluid system. The inclusions trapped at
200°C will be of either or both of the 2 fluid compositions
shown by the 2 red stars on the diagram. Intermediate
compositions cannot occur because the 2 fluids are immiscible.
(Trapping mixtures of these two immiscible fluids within the
same tiny fluid inclusion is highly improbable.) Because the
super-critical CO2-rich carbonic phase (which is
gaseous and buoyant) is often lost and not trapped in
inclusions, the original CO2 content of the fluid
cannot be determined from the remaining aqueous inclusions
trapped at 200°C. In this example, after escape of the gas phase
fluid, only the aqueous fluid will be trapped in fluid
inclusions and it will seem that the parent fluid was
homogeneous and had only a low CO2 content of about
0.05 mole fraction CO2. However this is incorrect
because the CO2 rich fluid has been lost before
trapping and is not present in any fluid inclusions.
Rarely, if the carbonic fluid bubbles are small
and not flushed out of the system by turbulence in the system,
the CO2 rich fluid may be trapped. These conditions
may allow the carbonic bubbles to adhere to growing crystal
surfaces which interferes with silica deposition and forms
carbonic fluid inclusions as silica is forced to deposit around
these bubbles. The bubbles would have to remain in place for a
long time to be encased in silica. This would give CO2-only
inclusions, despite actually forming from a heterogeneous,
dominantly aqueous, fluid mixture. Such fluid inclusions would
have CO2 contents of 90% and may well appear to be
totally carbonic at room temperature because the volume of the
condensed liquid water phase would be extremely small and exist
merely as an invisible film on the host mineral walls of the
inclusion. (A calculation of phase
volumes confirming this is here.) These same depositional
conditions may give rise to only few and/or only small aqueous
fluid inclusions if silica growth is slow and forms well ordered
quartz, lacking crystal defects. Such crystal defects are the
precursors of the cavities which become fluid inclusions. Small
fluid inclusions typically cannot be observed well enough to
study them and are overlooked or assumed to be unimportant
secondary inclusions. The failure to trap or to recognize these
aqueous inclusions leads to the false assumption that only CO2
fluids were present. These competing mechanisms of fluid
inclusion trapping act in mutual opposition and it cannot be
assumed that both fluid phases should be present in FIAs, and
the absence of either type of fluid inclusion in the sample does
not prove the absence of that type of parent fluid. The
misrepresentation of the parent fluid composition is merely a
function of selective trapping of the heterogeneous fluid phases
by mutually opposing trapping mechanisms.
The carbonic fluid inclusions may also suffer
post-entrapment water removal. The carbonic inclusions typically
have a very high internal pressure. The 0.9 X-CO2
inclusions trapped at 1 Kbar and 200 C in the above
example have a molar volume of about 50 cc/Mole (density
0.83 g/cc) and still have an internal pressure of more than 300
bar at 100 C and 1 bar room conditions. This pressure
differential could facilitate preferential diffusion or
migration of H2O out of the inclusion
because the size of H2O molecules is much less than
of CO2 molecules. This would result in more CO2
enriched, "water free" inclusions.
The apparent absence of aqueous inclusions in the
sample and the absence of any visible water within carbonic
fluid inclusions could be misinterpreted as deposition from a
non-aqueous CO2 fluid. However as seen in this
example, the parent fluid was in fact dominantly aqueous with a
composition of 0.95 mole fraction H2O and 0.05 mole
fraction CO2 at the trapping temperature. It would be
quite wrong to infer formation from CO2-only fluids,
despite the fact that only carbonic inclusions were observed in
the sample. An aqueous phase must have been present to transport
and deposit the quartz host mineral as silica is not
soluble in pure CO2 fluids, not even if they are
super-critical. (Liu,
W. et al. 2015)
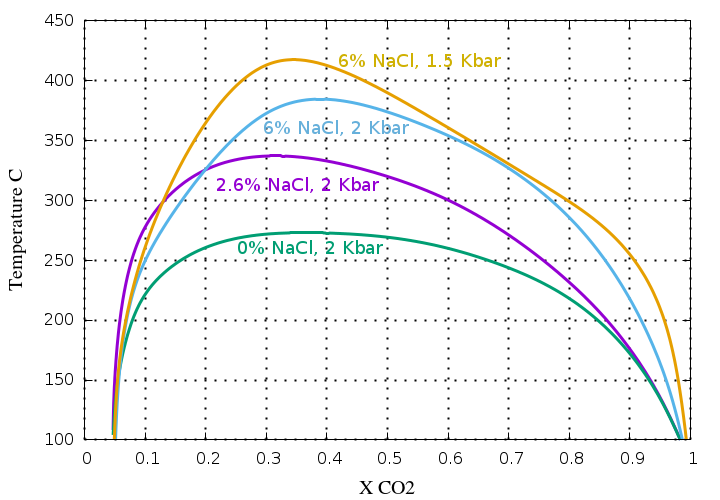
If NaCl (or other salts) are present in the fluid,
the CO2 immiscibility is greatly increased and
this plot shows the significant effect of salt on the solvus,
greatly increasing the immiscibility region.
The effect of CO2 on silica solubility
and inclusion formation
The solubility of silica in aqueous solution is
inversely dependent on the concentration of CO2 in
the fluid. Consequently as the fluid cools down to the
solvus temperature and partitions into two components,
ex-solving a CO2 rich phase, silica deposition
will be interrupted or will not commence until considerably more
cooling has occurred to compensate for the increased silica
solubility in the now CO2 depleted aqueous phase.
Many CO2 rich fluids will intersect the solvus and
undergo immiscibility separation during cooling, becoming
heterogeneous. There may be a complex fluid inclusion assemblage
due to this hiatus of silica deposition, or just heterogeneous
deposition from the aqueous phase after phase separation. The
gaseous CO2 phase is likely to be lost due to its
buoyancy and not trapped in fluid inclusions, so evidence of
this phase separation is unlikely to be preserved in fluid
inclusion assemblages.
This diagram shows the relationship between CO2
and quartz solubility with temperature in a 5 wt % NaCl
solution. The data is replotted from Thomas Monecke, Jochen
Monecke, and T. James Reynolds, The co-influence of CO2
on the solubility of quartz in single-phase hydrothermal fluids:
implications for the formation of stock-work veins in porphyry
copper deposits. Economic Geology, 2019, v. 114, no. 6,
pp. 1195–1206.
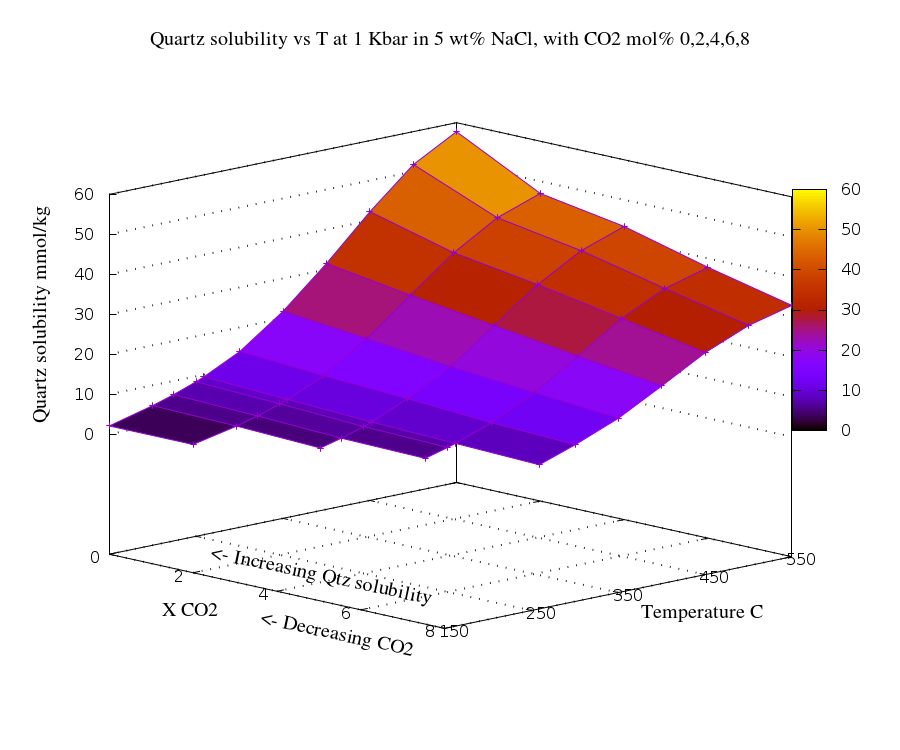
This is calculated data based on the density
model of Akinfiev and Diamond (2009), using the software
package Loner AP of R.J. Bakker
(http://fluids.unileoben.ac.at/Computer.html), which is based on
the thermodynamic equation of state of Duan et al. (1995).
The confusion over heterogeneous versus
homogeneous trapping
By default, everyone assumes that inclusions are
trapped from a homogeneous fluid, unless it is proven otherwise.
The criteria outlined by Ramboz et al. (1982) is used to
conclusively prove heterogeneous trapping. But failure to
satisfy these criteria does NOT prove that the fluid must have
been homogeneous.
The main problem is that the criteria assume
that inclusions trapped from heterogeneous systems will include
both of the conjugate pair fluids. This requirement ignores the
occurrence of disproportional trapping, which is normal in such
fluids. Roedder has explained the
importance of this issue which must not be ignored. The
failure to see both conjugate fluids in the trapped inclusion
assemblages is merely a function of selective trapping due to
the 2 different and mutually opposing mechanisms which are
involved in trapping either the aqueous or carbonic fluids
(bubbles). This is explained
here. Heterogeneous trapping is widespread in CO2-rich
systems but rarely recognized!
The Ramboz criteria
The Ramboz et al. (1982) criteria are intended to
be used to unambiguously identify heterogeneous trapping based
solely on observations in thin section.
- The two types of inclusions must occur in the
same regions of the same sample, and there must be good
evidence of their contemporaneous trapping:
This assumes that both of the conjugate
pair fluids are trapped, which is rarely the case.
Selective trapping, which is common, may result in only
one fluid being observed and this criteria cannot
disprove heterogeneous trapping.
- The two types of inclusions must homogenize at the same
temperature, or more realistically within the same range
of temperature (because trapping is not an instantaneous
and strictly isothermal-isobaric process):
Again, this assumes that both of the
conjugate pair fluids are present. The absence of one of
the conjugate fluids and inability to therefore measure
its homogenization temperature may merely be selective
trapping and cannot disprove
heterogeneous trapping.
- Upon heating the pressure difference is generally not
sufficient to allow the two types to decrepitate at very
different temperatures (unless their size and shape are
very different), so the pressures must reach the same
value (trapping pressure) at homogenization
temperature. Therefore, if one inclusion type decrepitates
before homogenizing, the other type must behave similarly: Again, this assumes that both of the conjugate
pair fluids are present. Furthermore it is known that CO2
rich fluid inclusions decrepitate at anomalously low
temperatures because the pressure in a gas-rich inclusion
increases according to the gas law equation, PV=nRT.
This will cause decrepitation long before the conjugate
aqueous inclusion decrepitates, as I have explained
here. This criteria is incorrect for CO2-rich
inclusions even if both conjugate fluids are present.
These criteria may be useful in proving the
existence of heterogeneous trapping, but its failure does not
prove the converse, i.e. that the fluid was homogeneous.
The following P-T plot shows solvus curves for 3 levels
of CO
2 and shows the extensive immiscibility field,
particularly so if even low levels of salt are present. The red
solvus curves are for pure H
2O with no salt, the
purple curves show the solvus of saline fluids. Only limited
data points are available to define the saline solvus curves.
The solvus curves are derived from the data and
3D plot of P-T-X-CO2
shown here and from the
salinity plot
above.
Homogenization to a liquid phase inclusion happens close to the
water liquidus, such as at the points shown by a black star. The
actual trapping temperature of inclusions depends on the
pressure and is along the isochore through the homogenization
point, shown as pale blue lines. It is often assumed that
trapping occurs from a homogeneous fluid, but in many cases
trapping occurs below the solvus curve and is actually the
aqueous phase of a separated immiscible fluid. Trapping in the
dark shaded area is unquestionably heterogeneous. For fluids
containing salt, trapping in the light shaded area may also be
heterogeneous. The fluid may be heterogeneous at pressures
up to more than 1 Kbar, which is about 4 Km depth, lithostatic
pressure. Many hydrothermal deposits form within this depth
range and fluid salinity and the observed inclusions must be the
aqueous fraction derived from a heterogeneous parent fluid. Even
fluids which homogenize at temperatures as high as 300 C could
be derived from a heterogeneous parent fluid.
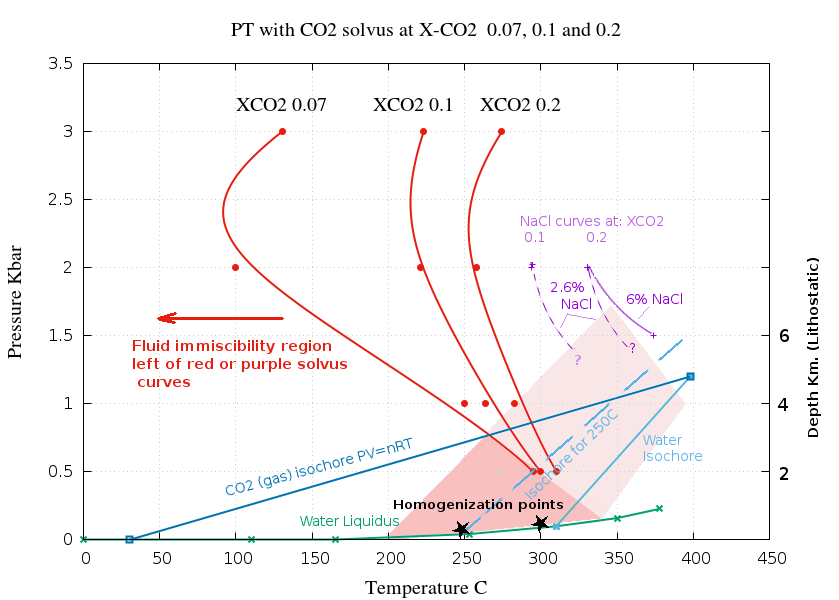
Many fluid inclusions from hydrothermal deposits
have homogenization temperatures below 300 C, formed at depths
less than 4 Km and contained enough salt that they must
have unmixed and be the result of heterogeneous trapping. The
absence of inclusions of both of the conjugate fluids is merely
the result of selective trapping because the buoyant gas phase
bubble is swept away before it can be silicified in place. Such
silicification is inhibited by the increase in quartz solubility
as CO2 exsolves further suppressing fluid
inclusion trapping. The aqueous phase is trapped by a completely
different mechanism to the gas phase which strongly favors
selective trapping of the aqueous phase inclusion without
trapping the conjugate fluid gas bubble.
Observations of the homogenization temperature
and salinity are enough to indicate the occurrence of
heterogeneous trapping, despite the absence of the related
conjugate fluid. It is wrong to assume homogeneous
trapping merely because no matching conjugate fluids are
observed in thin section. CO2 immiscibility exists to
pressures of more than 1 Kbar at depths exceeding 4 Km and
occurs in many hydrothermal fluid systems.
Conclusions
The extensive immiscibility between water and CO2
is often ignored during fluid inclusion studies merely because
it is difficult to identify the presence of these heterogeneous
systems based solely on observations of fluid inclusion
assemblages in thin sections of the samples. It is also wrong to
assume that the CO2-H2O heterogeneous
system is similar to the boiling water heterogeneous system.
(The immiscible H2O and CO2 phases behave
very differently to the miscible heterogeneous liquid-water /
water-vapour system documented in the literature.) It is often
assumed that inclusions trapped from heterogeneous fluid systems
must trap both component fluids giving bimodal FIAs, but this is
incorrect (long
since explained by E. Roedder, 1984) because the carbonic
fluid (a gas) is usually lost and not trapped in inclusions. It
is also assumed there should be a continuum of compositions
between the 2 end member aqueous and carbonic fluids. However
that would require trapping of mixtures of the immiscible fluids
within individual fluid inclusions, which is improbable. The
often assumed (Ramboz) criteria for identification of
heterogeneous trapping are inappropriate because they assume
both conjugate fluids are trapped, which is not the case. The
Ramboz criteria cannot disprove heterogeneous trapping and
therefore cannot prove homogenous trapping. It is also essential
to consider the transport and deposition of silica in order to
seal up fluid inclusions, something which is ignored by almost
all authors. Silica
is not transported in non-polar CO2 fluid, not
even if it is super-critical. And few authors consider the multiple
mechanisms of fluid inclusion trapping which may act in
mutual opposition to give extreme disproportional trapping from
heterogeneous fluids. The inverse
relation between quartz solubility and CO2 content
interrupts quartz deposition as CO2 exsolves,
complicating the understanding of FIAs.
Many (perhaps most) CO2 rich
hydrothermal fluids will undergo immiscible phase separation
during deposition of quartz and formation of fluid inclusions
and are hence the result of heterogeneous deposition. It is
incorrect to assume such fluids are homogenous unless proved
otherwise, and we should actually assume the inverse; that CO2
rich fluid inclusions are formed from heterogeneous fluids
unless proven to be solely formed from a homogeneous fluid.
The criteria proposed by Ramboz
et al. (1982) (and also
discussed above) to prove heterogeneous trapping do not
account for the occurrence of disproportional trapping which
is often extreme in CO2 rich fluid systems.
For CO2 rich fluids,
heterogeneous inclusion trapping is the more common normality
and the frequent unproven assumptions of deposition from
homogeneous fluids are incorrect.
CO2-only fluid inclusions are rare,
but may be trapped from heterogeneous aqueous, CO2-rich
fluids which have undergone phase separation into a
conjugate pair of fluids due to immiscibility. These
fluids may be incorrectly recognized because of
disproportional trapping of the separate phases. Such
heterogeneous fluids are very common (although difficult to
recognize in petrographic thin sections of samples) because
there is an extensive Pressure - Temperature - Composition
(P-T-X) region of immiscibility in the H2O-CO2-NaCl
system which includes most of the P-T-XCO2
region in which hydrothermal gold deposits form. (Gold
deposits are frequently associated with CO2 rich
fluids.) Depending on the fluid conditions such as flow rate
and turbulence and the rate of silica deposition, only the CO2
fluid may be trapped as fluid inclusions due to extremely
selective trapping. It is counter-intuitive to propose
the formation of CO2-only inclusions from CO2-only
fluid as such postulates fail to explain the essential
deposition of the host mineral phase, silica.
A proposed method to help identify
heterogeneous trapping of immiscible fluids is to observe the
shape of the carbonic inclusions. Rounded inclusions are
indicative of trapping as an immiscible gas bubble within a
liquid host fluid, proving heterogeneous trapping, as discussed here.
References
Claire Ramboz, Michel Pichavant and Alain
Weisbrod, Fluid immiscibility in natural processes: Use
and misuse of fluid inclusion data: II Interpretation of
fluid inclusion data in terms of immiscibility.
Chemical Geology, 37
(1982) 29---48
 Applied Mineral Exploration
Applied Mineral Exploration Discussion and research relevant to mineral
exploration.
Discussion and research relevant to mineral
exploration. 


